How does ABUS help cancer detection in women with dense breasts?
In average risk women with dense breasts, adding automated breast ultrasound (ABUS) to routine screening mammography increases the detection of breast cancer by 36-167%.
Table 4: Mammography Cancer Detection Rates (per 1,000) Alone and With ABUS
| Density | N | Mammography (Alone) | Mammography + ABUS | Rate Change | Rate Change (%) |
|---|---|---|---|---|---|
| Kelly et al. (2010)16 | 4,419 | 3.6 | 7.2 | 3.6 | +100% |
| Giuliono at el. (2013)17 | 3,418 | 4.6 | 12.3 | 7.7 | +167% |
| Brem et al. (2015)18 | 15,318 | 5.4 | 7.3 | 1.9 | +36% |
| Wilczek et al. (2016)19 | 1668 | 4.2 | 6.6 | 2.2 | +52% |
| (Weighted Avg) | 24,823 | 4.9 | 7.9 | 3.0 | +66% |
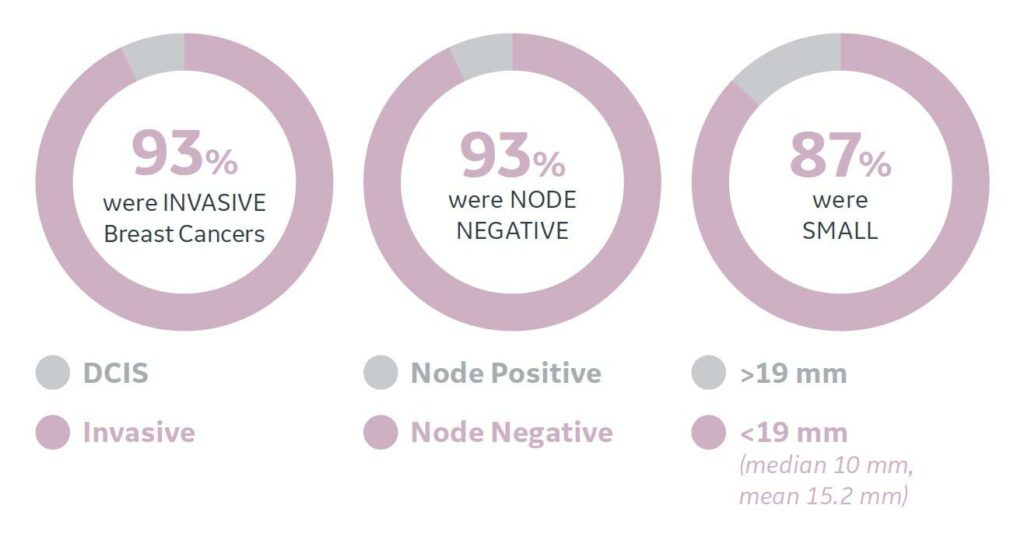
These cancers detected by ABUS tend to be small, invasive and node negative.
What is breast density?
Breast density is determined by the radiologist interpreting the mammogram and is assigned into one of four possible categories1 (see below). Breast density as shown on mammography depends on the extent of fibrous and glandular tissue in the breasts and has no correlation with findings on palpation.
Table 1: Mammographic Breast Density Classification
| Density | % of Breast Composed of Fibroglandular Tissue | ACR Category | % of Population² |
|---|---|---|---|
| Fatty | <25% | A | 10% |
| Scattered fibroglandular | 25 – 50% | B | 43% |
| Heterogenously dense | 51– 75% | C | 39% |
| Dense or Extremely dense | >75% | D | 8% |
How does breast density alter the ability of mammography to detect breast cancer?
Mammography alone is only 62-69% sensitive for the detection of breast cancer in women with heterogeneously dense or extremely dense breasts compared to 82-88% sensitive in women with lower breast density.
Table 2: Decreasing Mammography Sensitivity with Increased Breast Density
| Density | ACR Category | Sensitivity³ (%) |
|---|---|---|
| Fatty | A | 88% |
| Scattered fibroglandular | B | 82% |
| Heterogenously dense | C | 69% |
| Dense or Extremely dense | D | 62% |
What is the result of the decreased sensitivity of mammography in women with dense breasts?
Dense breast parenchyma can obscure small cancers (known as the masking effect)4. The risk of a patient presenting clinically with an interval cancer (between screening mammograms) is up to 18-fold higher comparing women at density extremes5. In the only series with sufficiently long-term follow-up to address mortality from breast cancer, Chiu et al.6 observed a 1.9-fold risk of breast cancer death (95%CI 1.26–2.91) among women with dense breasts after adjusting for other factors.
How is breast density related to a women’s risk of developing breast cancer?
Women with dense breasts have an increased risk of developing breast cancer that is independent to all other risks factors (such as their family history). The increased amount of epithelial and stromal elements present in dense breasts means a greater risk that a cancer may arise7. Women with heterogeneously dense breasts may be 1.2-1.5 times more likely to develop breast cancer where as women with extremely dense breasts may be 2.1-2.3 times more likely8-14. By comparison, having a 1st degree family member with breast cancer makes a women 1.8 or 3.3 times more likely to develop breast cancer in her lifetime depending on whether the family member was post or pre menopausal (respectively) when diagnosed15.
Table 3: Breast Cancer Risk Factors4-12
| Risk Factor | Category of Risk | Comparison Category | Relative Risk (RR) |
|---|---|---|---|
| Alcohol Intake | 2 drinks per day | Nondrinker | 1.2 |
| BMI | 80th percentile (age 55 or greater) | 20th percentile | 1.2 |
| Current Age | ≥65 yrs | < 65 yrs | 5.8 |
| Past History of Breast Cancer | Invasive breast cancer | No history of invasive breast cancer | 6.8 |
| Family History | 1st degree relative ≥50 yrs with postmenopausal breast cancer | No 1st, 2nd-degree relative with breast cancer | 1.8 |
| 1st degree relative with premenopausal breast cancer | No 1st, 2nd-degree relative with breast cancer | 3.3 | |
| 2nd degree relative with breast cancer | No 1st, 2nd-degree relative with breast cancer | 1.5 | |
| Breast Density | Heterogeneously dense (ACR Category C) | Average breast density | 1.2 - 1.5 |
| Extremely dense (ACR Category D) | Average breast density | 2.1 - 2.3 |
What are the risks of ABUS?
The two risks of ABUS for a patient are the increased risk of being recalled for supplemental imaging and an increased risk of ultimately undergoing a biopsy. The largest trial comparing mammography alone to mammography with supplementary ABUS (in average risk patients) showed that patients undergoing ABUS had an 89% increase of being recalled for additional imaging and a 93% increase rate of undergoing a breast biopsy18.
For patients coming to Insight Medical Imaging, this could potentially increase our current mammography recall rate from about 5% to 10%. Similarly, this could potentially increase the percentage of our screening patients ultimately undergoing biopsy from about 1% to 2%.
As a stand-alone test, ABUS had a lower false negative rate compared to mammography in women with dense breasts (15% vs. 27%, respectively)16. A very small but indeterminate number of cancers will be occult to both mammography and ABUS.
How should physicians incorporate ABUS into routine breast cancer screening?
All studies using ABUS to supplement screening mammography performed the tests concurrently at 1 year intervals16-19. Therefore for patients with dense breasts wishing to undergo screening mammography at one year intervals, yearly (concurrent) supplementary ABUS is most appropriate at present.
Patients with dense breasts that elect to undergo screening mammography every two years could undergo ABUS concurrent to mammography, alternating every year with mammography or have ABUS yearly.
What about using ABUS in women with non-dense breasts?
In average risk women with non-dense breasts, ABUS should not be used for supplemental screening for two main reasons. Firstly, mammography has good sensitivity for breast cancer in women with low (ACR category A/B) breast density due to the lack of masking effect3. Secondly, there is poor ultrasound contrast between lesions and the adjacent tissues in women with non-dense breasts, hindering lesion detection.
What about using ABUS in screening programs for women at high risk for breast cancer?
Current guidelines recommend annual screening with both MRI and mammography for women considered high risk for breast cancer irrespective of breast density (eg. women with BRCA gene mutation). ABUS can be as considered as another supplemental screening tool in certain clinical settings (eg. in women where MRI is contraindicated) but is an inferior substitute in high risk women as MRI has a higher cancer detection rate.
Why does UpToDate recommend against supplemental screening breast ultrasound?
The UpToDate chapters pertaining to breast ultrasound reference an article20 which make conclusions like, “Women who underwent [mammography and ultrasound] compared with those who underwent mammography alone had similar cancer detections rates (5.4 versus 5.5 per 1000) and similar interval cancer rates (1.5 versus 1.9 per 1000).” By selectively quoting this singular reference, they have ignored a much larger body of evidence (on much larger sample sizes) showing the exact opposite, namely that adding supplemental breast ultrasound to mammography improves breast cancer detection rates16,17,18,19.
UpToDate also quotes references stating that, “The addition of ultrasound to mammographic screening has yet to be shown to reduce breast cancer mortality21”. Invoking this line of thinking is however nonsensical as researchers would have to re-prove the entire paradigm of breast cancer screening to show that finding invasive cancers early at a node negative stage improves patient outcomes (morbidity and mortality). In the coming decade this data may become available but for now there is no plausible reason to think that finding early invasive will not improve breast cancer mortality.
References
- D’Orsi CJ, et al. Breast Imaging Reporting and Data System: ACR BI-RADS. 5th ed. Reston, Va: American College of Radiology, 2013.
- Pisano ED, et al. Digital Mammographic Imaging Screening Trial (DMIST) Investigators Group. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005 Oct 27;353(17):1773-83.
- Carney PA, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003 Feb 4;138(3):168-75.
- McCormack VA, dos Santos Silva I. Breast density and paren- chymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2006;15(6):1159–1169.
- Boyd NF, et al. Mammographic density and breast cancer risk: current understanding and future prospects. Breast Cancer Res. 2011; 13(6):223.
- Chiu SY, et al. Effect of baseline breast density on breast cancer incidence, stage, mortality, and screening parameters: 25-year follow-up of a Swedish mammographic screening. Cancer Epidemiol Biomarkers Prev. 2010; 19(5):1219–1228.
- Freer PE. Mammographic breast density: impact on breast cancer risk and implications for screening. Radiographics. 2015 Mar-Apr;35(2):302-15.
- Sickles EA. The use of breast imaging to screen women at high risk for cancer. Radiol Clin North Am 2010;48(5):859–878.
- Boyd NF, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007; 356(3):227-236.
- Byrne C, et al. Mammographic features and breast cancer risk: effects with time, age, and menopause status. J Natl Cancer Inst. 1995;87(21):1622-1629.
- Ursin G, et al. Mammographic density and breast cancer in three ethnic groups. Cancer Epidemiol Biomarkers Prev. 2003;12(4):332-338.
- Boyd NF, et al. Quantitative classification of mammographic densities and breast cancer risk: results from the Canadian National Breast Screening Study. J Natl Cancer Inst. 1995;87(9):670-675.
- Vacek PM, Geller BM. A prospective study of breast cancer risk using routine mammographic breast density measurements. Cancer Epidemiol Biomarkers Prev. 2004;13(5):715-722.
- Bertrand KA, et al. Mammographic density and risk of breast cancer by age and tumor characteristics. Breast Cancer Res. 2013;15(6):R104.
- Singletary SE. Rating the risk factors for breast cancer. Ann Surg. 2003 Apr;237(4):474-82.
- Kelly KM, et al. Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur Radiol. 2010 Mar;20(3):734-42.
- Giuliano V, Giuliano C. Improved breast cancer detection in asymptomatic women using 3D-automated breast ultrasound in mammographically dense breasts. Clin Imaging. 2013 May-Jun;37(3):480-6.
- Brem RF, et al. Assessing improvement in detection of breast cancer with three-dimensional automated breast US in women with dense breast tissue: the SomoInsight Study. Radiology. 2015 Mar;274(3):663-73.
- Wilczek B, et al. dding 3D automated breast ultrasound to mammography screening in women with heterogeneously and extremely dense breasts: Report from a hospital-based, high-volume, single-center breast cancer screening program. Eur J Radiol. 2016 Sep;85(9):1554-63.
- Lee JM, et al. Performance of Screening Ultrasonography as an Adjunct to Screening Mammography in Women Across the Spectrum of Breast Cancer Risk. JAMA Intern Med. 2019;179(5):658.
- Slanetz PJ, et al. Breast-density legislation–practical considerations. N Engl J Med. 2015 Feb;372(7):593-5.
Background
Biannual (every 6 month) surveillance for HCC in at risk groups has been shown to reduce mortality1. As such, surveillance programs are now recommended by many international liver guidelines2-5.
High-risk groups eligible for HCC surveillance include2-5:
- Cirrhosis, Child-Pugh class A and B
- Cirrhosis, Child-Pugh class C, awaiting liver transplant
- Noncirrhotic hepatitis B virus (HBV) patients with:
- Active hepatitis
- Family history of HCC
- African heritage
- Asian males > 40 years of age
- Asian females > 50 years of age
- Noncirrhotic hepatitis C virus (HCV) with advanced liver fibrosis (F3) (recommended only by the European Association for the Study of the Liver)
Cirrhosis patients are known to have an annual HCC incidence >/= 1.5%
HBV patients with a risk factor have an annual HCC incidence >/= 0.2%
Ultrasound for HCC Surveillance
Ultrasound performance is variable and dependent on patient related factors (ex: body habitus), imaging technique, and reference standard used6-8. One major study cites the sensitivity and specificity of ultrasound to be:
- Sensitivity
- All stages = 78%
- > 4 cm HCC = 85%
- 2-4 cm HCC = 62%
- < 2 cm HCC = 21%
- Specificity
- All patients = > 90%
Routine biannual (every 6 month) ultrasound surveillance has been shown to have the best cost-to-mortality benefit9-11.
Lesions < 1 cm are too small to reliably characterize on further imaging or biopsy and are more likely to be benign. Lesions which are >/= 1 cm warrant CT and MRI to further characterize. In some cases (LI-RADS 5), CT and/or MRI alone are sufficient for a confident diagnosis of HCC in appropriate patients.
HCC Surveillance Program
The recommended pathway for surveillance is as follows:
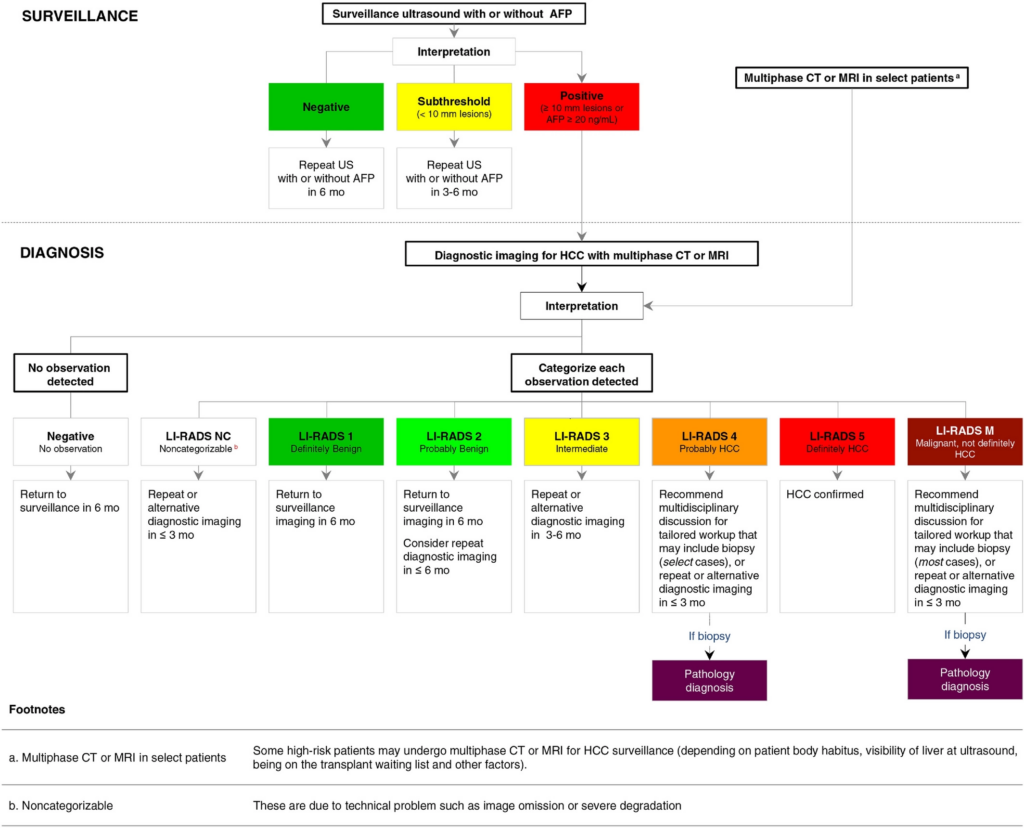
Reference: American Association for the Study of Liver Disease 2018 4.
More simply, in lesions with subthreshold or positive results, the imaging and biopsy pathway in Edmonton and area is as follows:
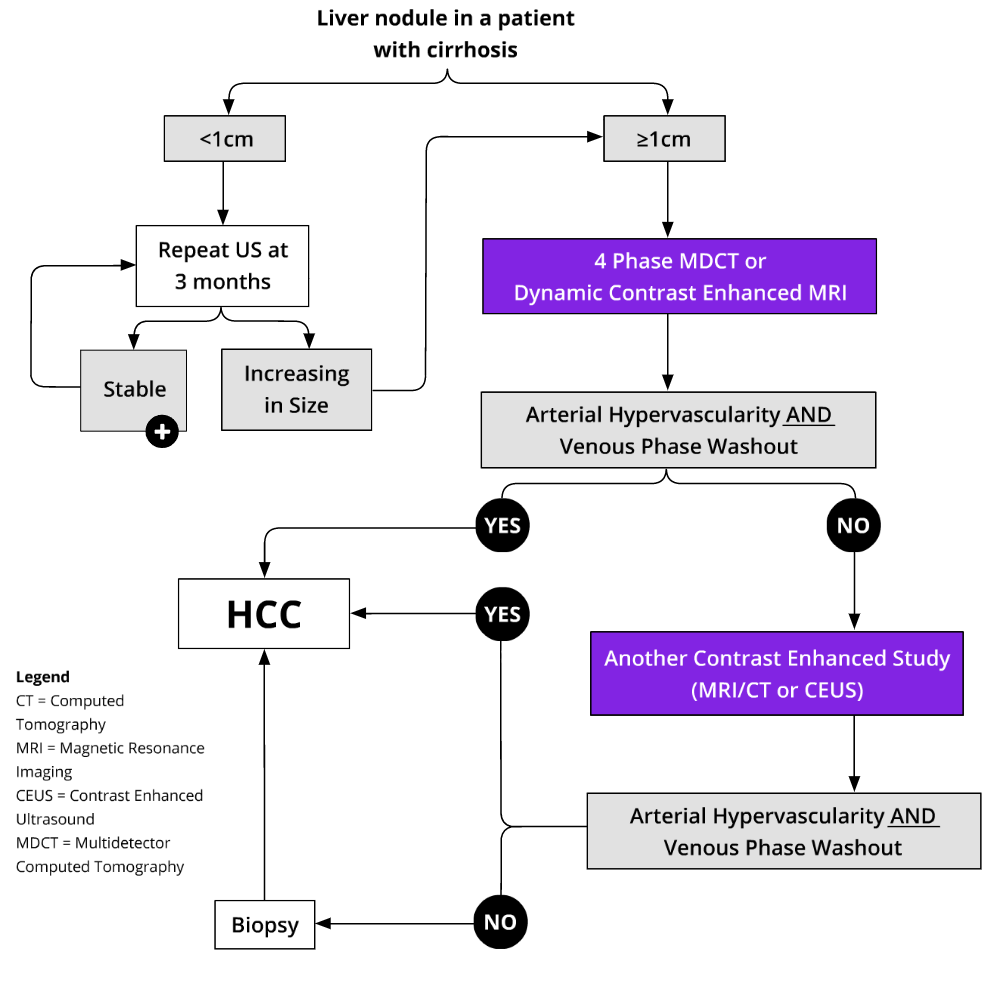
Reference: CirrhosisCare.ca14
HCC Surveillance – The Insight Medical Imaging Approach
Based on existing guidelines and local recommended best practice, the Insight Medical Imaging approach is as follows:
- In appropriate/eligible patients, submit a referral to Insight by selecting the “HCC Screening Program” option on the new General Requestions (available summer 2022). If your requisition does not include this check box, simply write “HCC Screening” or “HCC surveillance” in the history.
- The patient will be scheduled for a full abdomen ultrasound on their initial presentation if one is not available within the previous 3 months. In addition to routine assessment, additional components of a modified HCC surveillance ultrasound will be performed at this time which specifically includes assessment of:
- Liver – morphology and lesion assessment
- Spleen – size and volume
- Other
- Main portal vein assessment
- Peak systolic velocity
- Size (>13-16 mm) and velocity < 15 cm/s are suggestive of portal hypertension (although no consensus guideline values are available).
- Time averaged peak velocity (TAPV)
- Velocity < 15 cm/s has increased risk of thrombosis in high risk patients12.
- Peak systolic velocity
- Assessment of ascites
- Main portal vein assessment
- If no liver lesion is identified, the patient will automatically be re-scheduled for a surveillance study in 6 months.
- If a new lesion < 1 cm is identified, the patient will be re-scheduled for a follow-up surveillance study in 3 months. The lesion will be followed with surveillance studies every 3 months for 18 months. If no change occurs in this time, the patient will return to surveillance every 6 months.
- If a new lesion >/= 1 cm is identified, an MRI requisition will be submitted on your behalf and a follow-up surveillance ultrasound will be scheduled in 6 months.
- Please note, although the MRI requisition will be submitted on your behalf, we do ask that the referring physician follow up the results of the MRI with the patient directly. A recommendation will be provided separately on the MRI with respect to returning to surveillance, requiring biopsy, or requiring hepatobiliary consultation/referral to multi-disciplinary rounds.
- MRI reporting will be performed with the liver imaging reporting and data system (LI-RADS).
HCC Staging and Treatment
Staging and treatment of HCC in Alberta can be complicated and often requires discussion at multidisciplinary rounds. The current approach in Alberta is generally as follows:
Staging and Treatment – The Alberta HCC Algorithm
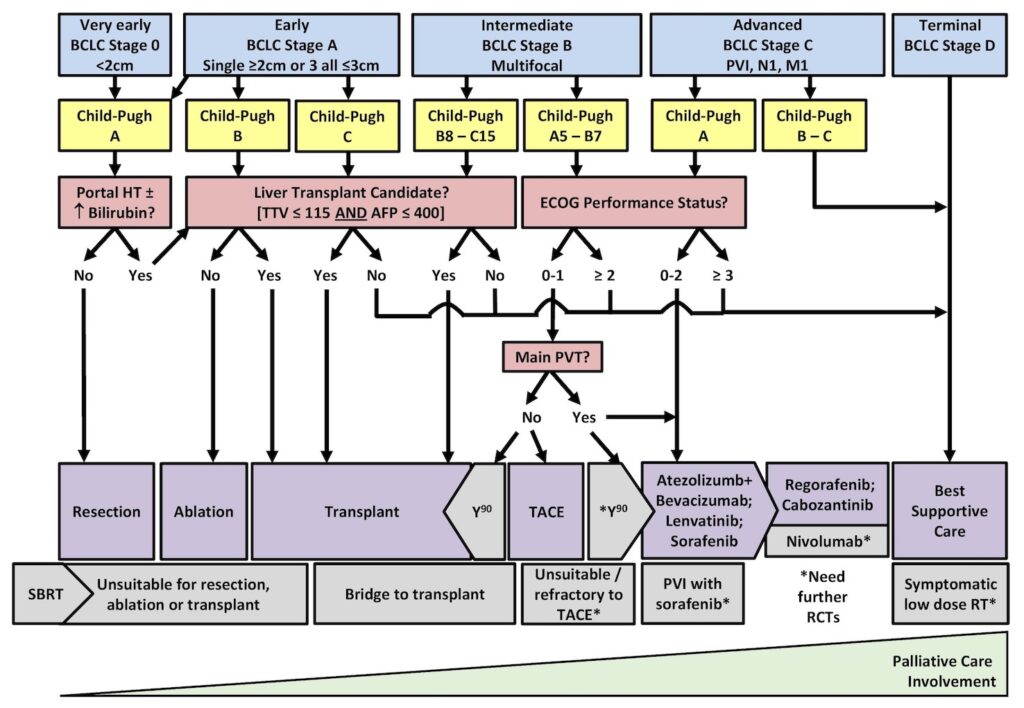
Reference: CirrhosisCare.ca14
References
- Zhang BH et al. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004;130(7):417.
- Clinical Practice Guidelines for Hepatocellular Carcinoma – The Japan Society of Hepatology 2009 update. Hepatol Res 2010;40 Suppl 1:2.
- EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69(1):182.
- Marrero JA et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the America Association for the Study of Liver Diseases. Hepatology 2018;68(2):723.
- Kanwal F et al. Surveillance for hepatocellular carcinoma: Current best practice and future direction. Gastroenterology 2019;157(1):54.
- Colli A et al. Accuracy of ultrasonography,spiral CT, magnetic resonance and alpha-fetoprotein in diagnosing hepatocellular carcinoma:A systematic review. Am J Gastroenterol 2006;101(3):513.
- Yu NC et al. CT and MRI improve detection of hepatocellular carcinoma, compared with ultrasound alone, in patients with cirrhosis. Clin Gastroenterol Hepatol 2001;9(2):161.
- Tzartzeva K et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: A meta-analysis. Gastroenterology 2018;154(6):1706.
- Sengal A et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther 2009;30(1):37.
- Santi V et al. Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. J Hepatol 2010;53(2):291.
- Trinchet JC et al. Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: a random trial comparing 3- and 6-month periodicities. Hepatology 2011;54(6):1987.
- Stine JG et al. Decreased portal vein velocity is predictive of the development of portal vein thrombosis: A matched case control study. Liver international 2018;38(1):94-101.
- Indiran V et al. Does coronal oblique length of spleen on CT reflect splenic index? Abdom Radiol 2017;42:1444-1448.
- https://cirrhosiscare.ca/treatment-provider/hepatocellular-carcinoma-hcp/. Accessed June 23, 2022.
- Colombo M. Surveillance for hepatocellular carcinoma in adults. UpToDate. Accessed June 23, 2022.
Insight Medical Imaging follows the Canadian Association of Radiologists (CAR) practice guidelines and technical standards for breast imaging and intervention. The CAR committee encourages a consensus-based approach for performing and interpreting breast imaging, interventional procedures, and best practice guidelines. The guidelines educate practitioners, radiologists, and technologists so there is a consistent understanding for what is considered best practice in the industry. These guidelines are not binding for practitioners, but rather evidence-informed principles of practice intended to generate a higher quality of radiological care for patients. The guidelines are in accordance with those published by the Canadian and American Cancer Societies, the National Comprehensive Cancer Network, American College of Radiology, and shared across the Alberta Society of Radiologists (ASR) and 2013 Alberta Toward Optimized Practice (TOP) screening recommendations.
Car and TOP Breast Cancer Mammography Screening Recommendations for Asymptomatic Women
39 Years & Under: Screening is not recommended because the incidence of breast cancer is low in this age group and there is no evidence for mortality reduction. [2]
40-49 Years: Health care providers must discuss the benefits and risks of screening in this age group. The ideal interval for this age group is less clear as there are a number of factors that can influence screening results such as breast density. Additionally, sojourn time and rapid tumor growth in younger women suggest shorter interval screening is ideal; the recommended screening interval is one year. [1][4]
50-74 Years: Mammography screening in this group has shown significant evidence of mortality reduction. Routine screening is recommended every two years (biennial). When compared to annual screenings in this age group, biennial screening preserves 80% of the benefit and has shown almost 50% fewer false positive results. [5]
75 Years & Older: women in this age group are at an increased risk for developing breast cancer, however the correlation between scanning and its benefits are not as significant. Health care providers should consider individual health factors when deciding whether to continue screening, however we encourage our patients to continue screening intervals for the rest of their life. The recommended interval for healthy patients is every two years (biennial).
*The Canadian Association of Radiologists (CAR) recommends 50-74 and 75+ age groups consider screening on an annual or biennial basis (one to two years). [6]
High Risk Population – Intensive Screening Exceptions
- Women who have one or two first degree relatives (sister, mother, daughter) with invasive breast cancer, exhibit symptoms, but do not meet the criteria for Medical Genetics referral, qualify for more intense screening measures.
-Talk to your doctor, but a baseline recommendation starts with annual screening mammography or breast ultrasound scans, starting 5-10 years younger than the youngest case in the family. (generally no earlier than age 25 and no later than age 40)
-Annual clinical breast examination starting at age 25.
- Women with a breast biopsy showing atypical hyperplasia or lobular carcinoma in situ and following surgical management to rule out invasive carcinoma.
-Annual mammography.
-Annual clinical breast examination.
- Women with a history of chest wall radiation at age 30 or younger.
-Annual mammography, breast ultrasound and breast screening MRI starting 5-10 years after radiation exposure. (no earlier than age 25 and no later than age 40)
-Annual Clinical breast examination.
Breast Imaging Reporting and Data System (BI-RADS) Assessment Categories
BI-RADS 0: Mammography: Incomplete*
Ultrasound and MRI: Incomplete*
*Need additional imaging evaluation and/or prior mammograms for comparison*
BI-RADS 1: Negative
BI-RADS 2: Benign
BI-RADS 3: Probably Benign
BI-RADS 4: Suspicious – 4A – Low suspicion for malignancy
4B – Moderate suspicion for malignancy
4C – High suspicion for malignancy
BI-RADS 5: Highly suggestive of malignancy
BI-RADS 6: Known biopsy-proven malignancy
References
- Buist DSM, Porter PL, Lehman C, Taplin SH, White E. (2004). Oct 6; 96(19):1432–40. Factors contributing to mammography failure in women aged 40-49 years. J. Natl. Cancer Inst.
- Canadian Task Force on Preventive Health Care, Tonelli M, Gorber S, Joffres M, Dickinson J, Singh H, et al. (2011). Nov 22; 183(17):1991–2001. Recommendations on screening for breast cancer in average-risk women aged 40–74 years. CMAJ.
- D’Orsi CJ, Sickles EA, Mendelson EB, Morris EA, et al. (2013) ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA, American College of Radiology. Retrieved from https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Bi-Rads
- Duffy SW, Chen HH, Tabar L, Fagerberg G, Paci E. (1996). Dec; 25(6):1139–45. Sojourn time, sensitivity and positive predictive value of mammography screening for breast cancer in women aged 40-49. Int J Epidemiol.
- Mandelblatt JS, Cronin KA, Bailey S, Berry DA, De Koning HJ, Draisma G, et al. (2009). Nov 17; 151(10):738–47. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann. Intern. Med.
- Shiela, C., Aldis, A., Causer, P., Crystal P., Mesurolle, B., Mundt, Y., Panu, N., … Wadden, N. (2016). CAR Practice Guidelines and Technical Standards for Breast Imaging and Intervention. Retrieved from https://car.ca/wp-content/uploads/Breast-Imaging-and-Intervention-2016.pdf
Insight Medical Imaging refers to Osteoporosis Canada’s 2010 Clinical Practice Guidelines and the Canadian Association of Radiologists 2013 Technical Standards for reporting 10 year fracture risk, Osteoporosis, and dexa scan results. These recommendations are merely guidelines, not rules, and ultimate procedure judgement resides with referring physician’s based on family history and unique circumstances presented in each case.
Bone Mineral Density Diagnostic Categories
| Patient Group | Category Name | T-Score Value | Z-Score Value |
|---|---|---|---|
| 50 Years and older | Normal | >= -1.0 | |
| Low bone mass (Osteopenia) | Between -1 and -2.5 | ||
| Osteoporosis | <= -2.5 | ||
| 49 years and younger | Within expected range for age | > -2.0 | |
| Below expected range for age | <= -2.0 |
For Adults 50 years and older:
Diagnostic category is determined using the lowest T-score for the lumbar spine, total hip, femoral neck, 33% radius, and total body.
For Adults 18 to 49 years old:
Diagnostic category is determined using the lowest Z-score for the lumbar spine, total hip, femoral neck, 33% radius, and total body.
For Adolescents 17 years and younger:
Diagnostic category is determined using the lowest adjusted Z-spine for the lumbar spine and total body. Z-scores require adjustment for one of more of height, weight, body mass index, bone area, bone age, pubertal stage, and lean body mass.
Determining a 10-year Absolute Fracture Risk
- Determine patient’s gender and identify row closest to patients age.
- Determine fracture risk category by using the femoral neck T-score.
- If your patient’s age is between rows, infer the T-score thresholds.
- If either the fragility fracture history or glucocorticoid history are positive, bump the patient in to the next highest risk category.
- Fracture risk is high regardless of the CAROC result if:
- Both fragility fracture history after age 40 years and glucocorticoid history are positive
- Glucocorticoid history is considered positive if prednisone or prednisone equivalents was in use at a dose > 7.5 mg/day for more than 90 days in the previous 12 months
- Patient’s with hypoadrenalism on replacement glucocorticoids should not be considered to have a positive glucocorticoid history for fracture risk evaluation regardless of the dose.
- There has been a fragility hip, vertebral, or more than two fragility fractures after age 40
- If the fracture risk category is low after the previous steps, the lumbar spine T-score is considered.
If the lumbar spine T-score is <-2.5, risk is increased to moderate
CAROC 10 Year Fracture Risk for Women (2010)
| Femoral Neck T-Score | |||
|---|---|---|---|
| Age (Years) | Low Risk (<10%) | Moderate Risk (10% - 20%) | High Risk (>10%) |
| 50 | Greater than -2.5 | -2.5 to -3.8 | Less than -3.8 |
| 55 | Greater than -2.5 | -2.5 to -3.8 | Less than -3.8 |
| 60 | Greater than -2.3 | -2.3 to -3.7 | Less than -3.7 |
| 65 | Greater than -1.9 | -1.9 to -3.5 | Less than -3.5 |
| 70 | Greater than -1.7 | -1.7 to -3.2 | Less than -3.2 |
| 75 | Greater than -1.2 | -1.2 to -2.9 | Less than -2.9 |
| 80 | Greater than -0.5 | -0.5 to -2.6 | Less than -2.6 |
| 85 | Greater than +0.1 | +0.1 to -2.2 | Less than -2.2 |
CAROC 10 Year Fracture Risk for Men (2010)
| Femoral Neck T-Score | |||
|---|---|---|---|
| Age (Years) | Low Risk (<10%) | Moderate Risk (10% - 20%) | High Risk (>10%) |
| 50 | Greater than -2.5 | -2.5 to -3.9 | Less than -3.9 |
| 55 | Greater than -2.5 | -2.5 to -3.9 | Less than -3.9 |
| 60 | Greater than -2.5 | -2.5 to -3.7 | Less than -3.7 |
| 65 | Greater than -2.4 | -2.4 to -3.7 | Less than -3.7 |
| 70 | Greater than -2.3 | -2.3 to -3.7 | Less than -3.7 |
| 75 | Greater than -2.3 | -2.3 to -3.8 | Less than -3.8 |
| 80 | Greater than -2.1 | -2.1 to -3.8 | Less than -3.8 |
| 85 | Greater than -2.0 | -2.0 to -3.8 | Less than -3.8 |
For an additional representation of the diagnosis process to determine a patient’s 10-year absolute fracture risk, please refer to Appendix 5, page 17, of Osteoporosis Canada’s step by step guide.
References
- Siminoski, K., O’Keeffe, M., Brown, JP., Burrell, S., Coupland, D., Dumont, M., … Levesque, J. (2013). CAR Technical Standards for Bone Mineral Densitometry Reporting. Retrieved from https://car.ca/wp-content/uploads/Technical-Standards-for-Bone-Mineral-Densitometry-Reporting-2013.pdf
- Papaioannou, A et al. (2010). 2010 Clinical Practice Guidelines for the Diagnosis and Management of Osteoporosis in Canada. Retrieved from http://www.osteoporosis.ca/multimedia/pdf/Quick_Reference_Guide_October_2010.pdf
2012 Canadian Association of Radiologists Diagnostic Imaging Referral Guidelines
The 2012 CAR guidelines are based on expert opinion or case studies, intended to assist physicians in the decision-making process regarding appropriate imaging studies for specific cases. These evidence-informed guidelines are not intended to restrict or diminish the freedom of practising physicians to order imaging studies for their patients for whom they have the ultimate responsibility. Furthermore, discussion between the radiologist and referring physician must always take precedence and is encouraged by all industry professionals at Insight Medical Imaging.
CAR referral Guides (PDF format)
Section A: Central nervous system
Section B: Head and neck
Section C: Spine
Section D: Musculoskeletal system
Section E: Cardiovascular
Section F: Thoracic
Section G: Gastrointestinal system
Section H: Urological, adrenal and genitourinary systems
Section I: Obstetrics and gynaecology
Section J: Trauma
Section K: Cancer
Section L: Pediatrics
Section M: Breast Disease
For more information visit the Canadian Association of Radiologists website.

